Eisai Co Ltd and Biogen Inc have announced that their experimental Alzheimer’s drug significantly slowed cognitive and functional decline in the early stages of the disease in a large trial of 1,800 patients, which is a rare success in this field.
The drug, lecanemab, slowed progress of the brain-wasting disease by 27% compared with a placebo, meeting the study’s main goal, and potentially offering hope for patients and their families desperate for an effective treatment, the pharma companies said.
“It’s not a huge effect, but it’s a positive effect,” said Ronald Petersen, director of the Mayo Clinic Alzheimer’s Disease Research Center in Rochester, Minnesota.
Japan’s Eisai, leader of the 50-50 partnership’s lecanemab program, is seeking an FDA approval for which the decision is expected in early January.
The company said it will also seek authorization in Japan and Europe during its current fiscal year, ending March 31.
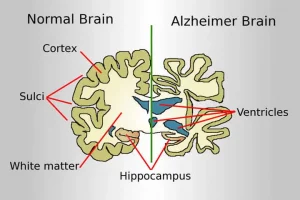
Eisai said results from the trial prove the longstanding theory that removal of sticky deposits of a protein called amyloid beta from the brains of people with early Alzheimer’s can delay progress of the debilitating disease.
The lecanemab data suggest “a potentially new multi-billion dollar franchise,” Jefferies analyst Michael Yee said in a research note.
Lecanemab, like the partners’ previous drug Aduhelm, is an intravenous antibody designed to remove amyloid deposits. Unlike Aduhelm, lecanemab targets forms of amyloid that have not yet clumped together.
“If you can slow a disease by almost 30% that’s fantastic. This is what we have been looking for,” said Dr. Jeff Cummings, director of the Chambers-Grundy Center for Transformative Neuroscience at the University of Nevada Las Vegas.
However, the so-called amyloid hypothesis has been challenged by some scientists, particularly after the U.S. Food and Drug Administration’s controversial approval of Aduhelm in 2021 based on its plaque-clearing ability rather than proof that it helped slow cognitive decline.
Aduhelm was the first new Alzheimer’s drug approved in 20 years after a long list of failures for the industry.