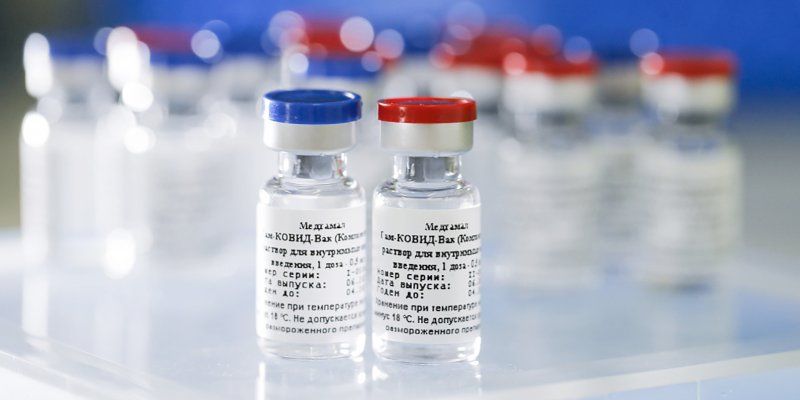
India-Russia ties have got a booster dose in the healthcare sector with New Delhi approving the Sputnik V vaccine for production in the country. The first shots of the Russian vaccine are likely to be rolled out in India as early as next month.
"Our cooperation in the health sector has added another dimension to the India-Russia strategic partnership. Simultaneously, it provides additional capacity to our global vaccine efforts and consolidates India-Russia cooperation in the health sector. This news has been well-received in Russia," India’s ambassador to Russia, Bala Venkatesh Varma told India Narrative.
The development comes as a major boost in the global war against Cvoid-19 at a time when there is an acute shortage of vaccines worldwide.
The Sputnik V vaccine is likely to be rolled out in May in India, Kirill Dmitriev chief of the Russian Direct Investment Fund (RDIF) indicated in an interview on Tuesday.
“I think it could be even earlier. We believe that there will be some quantities in India in May and we are looking at first half of May for delivery of the vaccine and then it will be increased in its volume quite a bit every month after that,” India Today quoted him as saying in an interview.
“So Sputnik will be India, based on the approvals, as early as late April but given the pace of the things it may be first half of May,” Dmitriev added.
He said the capacity would be ramped up and starting from July tens of millions of Sputnik vaccines will be produced in India for the local as well as export markets. Dmitriev said more than 500 million doses or more are expected to be produced in India.
India is poised to become a major hub for producing the Sputnik V vaccine with five Indian companies having signed agreements for the production of the vaccine. The total capacity works out to around 950 million doses a year.
Panacea Biotec was the fifth Indian company in the run-up to the Russian foreign minister’s visit to sign an agreement for producing 100 million doses of the Sputnik V vaccine. Three other Indian companies have signed agreements in recent weeks to roll out the vaccine. These include Virchow Biotech and Stelis Biopharma for 200 million doses each and for Gland Pharma for the manufacture of 252 million shots annually.
The approval for production and use of the Sputnik V vaccine in India was given by the government’s Subject Expert Committee (SEC) on Monday. India's drugs regulator will give the final go-ahead for the rollout but after the SEC clearance this is now a mere formality.
It would be the third COVID-19 vaccine approved in India, after AsztraZeneca’s Covishield being produced at the Pune-based Serum Institute of India, and Hyderabad-based Bharat Biotech’s home-grown Covaxin developed in partnership with the Indian Council of Medical Research.
Dr Reddy's Laboratories had last week sought the government's approval for the vaccine to be used in India. The Russian Direct Investment Fund (RDIF) partnered with Dr Reddy's in September 2020 to conduct clinical trials of Sputnik V in India.
The issue of expediting approvals for the production and distribution of the Sputnik V vaccine had figured at the talks between India’s foreign minister S. Jaishankar and his Russian counterpart Sergey Lavrov in Delhi on April 6.
The Russian vaccine, developed by Moscow’s Gamaleya Institute has an efficacy of 91.6% based on the interim analysis of phase III clinical trials, which included data from 19,866 volunteers in Russia and also carried out Phase III clinical trials in the UAE, India, Venezuela and Belarus, according to the Sputnik-V website.
The Sputnik V vaccine has already been approved for use in more than 50 countries. The vaccine has been used extensively in Russia.
Sputnik-V is an affordable vaccine and can be "stored at a temperature of +2 to +8°C in its solid form,” according to RDIF.